Enzyme Regulation
CHEMISTRY, BIOCHEMISTRY, PHARMACOLOGY, PROTEIN SURFACE MIMETICS, GPCR REGULATION, ENZYME REGULATION, ANTI-INFLAMMATORY RESEARCH, ANTI-INFECTIVES RESEARCH, ANTI-CANCER RESEARCH, NEURODEGENERATIVE RESEARCH, CARDIOVASCULAR RESEARCH
We use computer-assisted methods to design small organic compounds that can bind to enzymes in substrate-binding grooves (competitive inhibitors) or at more remote sites (non-competitive inhibitors) thereby preventing or down-regulating enzyme activity in vitro and in animal models of disease. We are experts in developing inhibitors of enzymes, including proteases, histone deacetylases, phospholipases, complement enzymes, viral and inflammatory enzymes. Some examples are inhibitors of :
1) NS2B-NS3 proteases from Flaviviruses such as Dengue and West Nile Viruses.
2) Aspartyl proteases (e.g. HIV protease, Schistosomal Cathepsin D, Plasmepsins).
3) Complement serine proteases such as C2, C3 convertase, Factor B.
4) Cysteine proteases (e.g. Caspase 1 (ICE), Caspases 3, 8, Cathepsins B,K and S).
5) Histone deacetylases (e.g. HDAC1, 4, 5, 6, 7).
6) Phosphlipases A2 (e.g. pla2g2a, pla2g5).
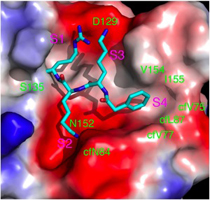

Figure. Inhibitors of Proteases: (Left) : Antiviral protease inhibitor (Ki 9 nM) of the heterodimeric NS2B-NS3 protease from West Nile Virus docked (GOLD) into the active site of the protease (Stoermer et. al, 2008) . (Right) Non-covalent Inhibitor of Caspase-1 that blocks formation of interleukin 1 beta.
PUBLICATIONS
General Enzyme Inhibitors
Comparing sixteen scoring functions for predicting biological activities of ligands for protein targets. Xu W, Lucke AJ, Fairlie DP. J Mol Graph Model 2015, 57, 76-88.
Proteases Universally Recognize Beta Strands In Their Active Sites. Tyndall, J. D. A.; Nall, T.; Fairlie, D. P. Chem. Reviews 2005, 105, 973-1000. [>100 citations]
Protease Inhibitors in the Clinic. Abbenante, G.; Fairlie, D. P. Medicinal Chemistry 2005, 1, 71-104. [>100 citations]
Conformational Selection Of Inhibitors and Substrates By Proteolytic Enzymes : Implications for Drug Design and Polypeptide Processing. Fairlie, D. P.; Tyndall, J. D. A.; Reid, R. C.; Wong, A. K.; Abbenante, G.; Scanlon, M. J.; March, D. R.; Bergman, D. A.; Chai, C. L. L.; Burkett, B. A. J. Med. Chem. 2000, 43, 1271-1281. [> 100 citations]
Protease Inhibitors: Current Status and Future Prospects. Leung D, Abbenante G, Fairlie DP. J. Med. Chem. 2000, 43, 305-341. [>600 citations]
Conformational Homogeneity In Molecular Recognition By Proteolytic Enzymes. Tyndall, J.; Fairlie, D. P. J. Molecular Recognition 1999, 12, 363-370.
Targeting HIV-1 protease: a test of drug-design methodologies. West ML, Fairlie DP. Trends Pharmacol Sci. 1995, 16, 67-75. [>100 citations]
Aspartic Protease Inhibitors
Crystal Structures of Highly Constrained Substrate and Hydrolysis Products Bound to HIV-1 Protease. Implications for Catalytic Mechanism. Tyndall, J. D. A.; Pattenden, L. K.; Reid, R. C.; Hu, S.-H.; Alewood, D.; Alewood, P. F.; Walsh, T.; Fairlie, D. P.; Martin, J. L. Biochemistry 2008, 47, 3736-44.
Countering cooperative effects in protease inhibitors using constrained beta strand-mimicking templates in focussed combinatorial libraries. Reid, R. C.; Pattenden, L. K.; Tyndall, J. D. A.; Martin, J. L.; Walsh, T.; Fairlie, D. P. J. Med. Chem. 2004, 47, 1641-51.
Beta strand mimicking macrocyclic amino acids. Templates for protease inhibitors with antiviral activity. Glenn, M. P.; Pattenden, L. K.; Reid, R. C.; Tyssen, D. P.; Tyndall, J. D. A.; Birch, C. J.; Fairlie, D. P. J. Med. Chem. 2002, 45, 371-381.
Synthesis, Stability, Antiviral Activity, and Protease-Bound Structures of Substrate-Mimicking Constrained Macrocyclic Inhibitors of HIV-1 Protease. Tyndall, J. D. A.; Reid, R. C.; Tyssen, D. P.; Jardine, D. K.; Todd, B.; Passmore , M.; March, D. R.; Pattenden, L. K.; Bergman, D. A.; Alewood, D.; Hu, S-H.; Alewood, P. F.; Birch, C. J.; Martin, J. L.; Fairlie, D. P. J. Med. Chem. 2000, 43, 3495-3504.
Molecular recognition of macrocyclic peptidomimetic inhibitors by HIV-1 protease. Martin JL, Begun J, Schindeler A, Wickramasinghe WA, Alewood D, Alewood PF, Bergman DA, Brinkworth RI, Abbenante G, March DR, Reid RC, Fairlie DP. Biochemistry. 1999, 38, 7978-88.
Substrate-Based Cyclic Peptidomimetics of Phe-Ile Val That Inhibit HIV-1 Protease Using A Novel Enzyme-Binding Mode. March, D. R.; Abbenante, G.; Bergman, D. A.; Brinkworth, R. I.; Wickramasinghe, W. A.; Begun, J.; Martin, J. L.; Fairlie, D. P. J. Am. Chem. Soc. 1996, 118, 3375-3379.
Regioselective Structural and Functional Mimicry of Peptides. Design of Hydrolytically Stable Cyclic Peptidomimetic Inhibitors of HIV-1 Protease. Abbenante, G.; March, D. R.; Bergman, D. A.; Hunt, P. A.; Garnham, B.; Dancer, R. J.; Martin, J. L.; Fairlie, DP. J. Am. Chem. Soc. 1995, 117, 10220-10226.
Serine Protease Inhibitors
Membrane-anchored Serine Protease Matriptase Is a Trigger of Pulmonary Fibrogenesis. Bardou O, Menou A, François C, Duitman JW, von der Thüsen JH, Borie R, Sales KU, Mutze K, Castier Y, Sage E, Liu L, Bugge TH, Fairlie DP, Königshoff M, Crestani B, Borensztajn KS. Am J Respir Crit Care Med 2016,193, 847-60.
Product release is rate-limiting for catalytic processing by the Dengue virus protease. Shannon AE, Pedroso MM, Chappell KJ, Watterson D, Liebscher S, Kok WM, Fairlie DP, Schenk G, Young PR. Sci Rep 2016, 6, 37539.
Simultaneous uncoupled expression and purification of the Dengue virus NS3 protease and NS2B co-factor domain. Shannon AE, Chappell KJ, Stoermer MJ, Chow SY, Kok WM, Fairlie DP, Young PR. Protein Expr Purif 2016, 119, 124-129.
An interaction between the methyltransferase and RNA dependent RNA polymerase domains of the West Nile virus NS5 protein. Tan CS, Hobson-Peters JM, Stoermer MJ, Fairlie DP, Khromykh AA, Hall RA. J Gen Virol 2013, 94, 1961-1971.
Complement Factor C2, Inhibiting A Latent Serine Protease In The Classical Pathway Of Complement Activation. Halili MA, Ruiz-Gómez G, Le GT, Abbenante G, Fairlie DP. Biochemistry 2009, 48, 8466–8472.
Structure activity relationships for substrate based inhibitors of human complement factor B. Ruiz-Gómez, G.; Lim, J.; Halili, MA; Le, GT; Madala, PK; Abbenante G.; Fairlie DP J Med Chem 2009, 52, 6042-6052.
Potent cationic inhibitors of West Nile virus NS2B/NS3 protease with serum stability, cell permeability and antiviral activity. Stoermer MJ, Chappell KJ, Liebscher S, Jensen CM, Gan CH, Gupta PK, Xu WJ, Young PR, Fairlie DP. J Med Chem. 2008, 51, 5714-21.
Profiling the enzymatic properties and inhibition of human complement factor B. Le, G.T.; Abbenante, G.; Fairlie, D.P. J. Biol. Chem. 2007, 282, 34809-34816.
Insights to Substrate Binding and Processing by West Nile Virus NS3 Protease :through Combined Modelling, Protease Mutagenesis, and Kinetic Studies. Chappell, K. J.; Stoermer, M. J.; Fairlie, D. P.; Young, P.R. J. Biol. Chem. 2006, 281, 38448-58.
Site-directed Mutagenesis and Kinetic Studies of the West Nile Virus NS3 Protease Identify Key Enzyme-Substrate Interactions. Chappell, K. J.; Nall, T.; Stoermer, M.J.; Fang, N.-X.; Tyndall, J.D.A.; Fairlie, D.P.; Young, P.Y. J. Biol. Chem. 2005, 280, 2896-2903.
Activity Of Recombinant Dengue 2 Virus NS3 Protease In The Presence Of NS2B Cofactor, Small Peptide Substrates, And Inhibitors. Leung, D.; Schroder, K.; White, H.; Fang, N.-X.; Stoermer, M. J.; Abbenante, G.; Martin, J. L.; Young, P.; Fairlie, D. P. J. Biol. Chem. 2001, 276, 45762-45771. [>100 citations]
Cysteine Protease Inhibitors
Non-covalent tripeptidylbenzyl and cyclohexyl amine inhibitors of the cysteine protease caspase-1. Löser R, Abbenante G, Madala PK, Halili M, Le GT, Fairlie DP. J. Med. Chem. 2010, 53, 2651-2655.
Organic azide inhibitors of cysteine proteases. Le, G.T.; Abbenante,G.; Madala, P.K.; Hoang, H.N.; Fairlie, D.P. J. Am. Chem. Soc. 2006, 128, 12396-12397.
Phospholipase A2 Inhibitors
An inhibitor of pla2g2a modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Iyer A, Lim J, Poudyal H, Reid RC, Suen JY, Webster J, Prins JB, Whitehead JP, Fairlie DP*, Brown L*. Diabetes 2012, 61, 2320-2329.
Gregory LS, Kelly WL, Reid RC, Fairlie DP, Forwood MR. Inhibitors of cyclooxygenase-2 and phospho-lipase A2 preserve bone architecture following ovariectomy in adult rats Bone 2006, 39, 134-142.
Levick S, Loch D, Rolfe B, Reid RC, Fairlie DP, Taylo SM, Brown L. Anti-fibrotic activity of a group IIa secretory phospholipase A2 inhibitor in a rat model of cardiac fibrosis. J. Immunol. 2006, 176, 7000-7.
Arumugam TV, Arnold N, Proctor LM, Newman M, Reid RC, Hansford KA, Fairlie DP, Shiels IA, Taylor SM. Comparative protection against rat intestinal reperfusion injury by a new inhibitor of sPLA2, COX-1 and COX-2 selective inhibitors, and an LTC4 receptor antagonist. Br. J. Pharmacol. 2003, 140, 71-80.
D-Tyrosine As A Chiral Precursor To Potent Inhibitors Of Human Non-Pancreatic Secretory Phospholipase A2 (IIa) With Anti-Inflammatory Activity. Hansford, K. A.; Reid, R. C.; Clark, C. I.; Tyndall, J. D. A.; Whitehouse, M. W.; Guthrie, T.; McGeary, R. P.; Schafer, K.; Martin, J. L.; Fairlie D. P. ChemBioChem. 2003, 4, 181-185.
Kinase Inhibitors
Identification and characterization of bi-thiazole-2,2'-diamines as kinase inhibitory scaffolds. Ngoei KR, Ng DC, Gooley PR, Fairlie DP, Stoermer MJ, Bogoyevitch MA. Biochim Biophys Acta. 2013, 1834,1077-1088.
A new paradigm for protein kinase inhibition : Blocking phosphorylation without targeting ATP binding. Bogoyevitch, M.A.; Fairlie, D.P. Drug Discovery Today 2007, 12, 622-633. [>100 citations]
Histone Deacetylase Inhibitors
Inhibitors of class I histone deacetylases attenuate thioacetamide-induced liver fibrosis in mice by suppressing hepatic type 2 inflammation. Loh Z, Fitzsimmons RL, Reid RC, Ramnath D, Clouston A, Gupta PK, Irvine KM, Powell EE, Schroder K, Stow JL, Sweet MJ, Fairlie DP, Iyer A. Br J Pharmacol 2019, 176, 3775-3790.
Identification of brain metastasis genes and therapeutic evaluation of histone deacetylase inhibitors in a clinically relevant model of breast cancer brain metastasis. Kim SH, Redvers RP, Chi LH, Ling X, Lucke AJ, Reid RC, Fairlie DP, Martin ACBM, Anderson RL, Denoyer D, Pouliot N. Dis Model Mech 2018, 11(7). pii: DMM034850.
Lysine Deacetylases and Regulated Glycolysis in Macrophages. Shakespear MR, Iyer A, Cheng CY, Das Gupta K, Singhal A, Fairlie DP, Sweet MJ. Trends Immunol 2018, 39, 473-488.
Insecticidal activities of histone deacetylase inhibitors against a dipteran parasite of sheep, Lucilia cuprina. Bagnall NH, Hines BM, Lucke AJ, Gupta PK, Reid RC, Fairlie DP, Kotze AC. Int J Parasitol Drugs Drug Resist 2017, 7, 51-60.
Effect of clinically approved HDAC inhibitors on Plasmodium, Leishmania and Schistosoma parasite growth. Chua MJ, Arnold MS, Xu W, Lancelot J, Lamotte S, Späth GF, Prina E, Pierce RJ, Fairlie DP, Skinner-Adams TS, Andrews KT. Int J Parasitol Drugs Drug Resist 2017, 7, 42-50.
Histone deacetylases (HDAC) in physiological and pathological bone remodelling. Cantley MD, Zannettino ACW, Bartold PM, Fairlie DP, Haynes DR. Bone 2017, 95, 162-174.
Histone deacetylases in monocyte/macrophage development, activation and metabolism: refining HDAC targets for inflammatory and infectious diseases. Das Gupta K, Shakespear MR, Iyer A, Fairlie DP, Sweet MJ. Clin Transl Immunology 2016, 5(1), e62.
Histone deacetylase enzymes as drug targets for the control of the sheep blowfly, Lucilia cuprina. Kotze AC, Hines BM, Bagnall NH, Anstead CA, Gupta P, Reid RC, Ruffell AP, Fairlie DP. Int J Parasitol Drugs Drug Resist. 2015, 5, 201-208.
Histone Deacetylase Inhibitors Promote Mitochondrial Reactive Oxygen Species Production and Bacterial Clearance by Human Macrophages. Ariffin JK, das Gupta K, Kapetanovic R, Iyer A, Reid RC, Fairlie DP, Sweet MJ. Antimicrob Agents Chemother 2015, 60, 1521-1529.
Profiling the anti-protozoal activity of anti-cancer HDAC inhibitors against Plasmodium and Trypanosoma parasites. Engel JA, Jones AJ, Avery VM, Sumanadasa SD, Ng SS, Fairlie DP, Skinner-Adams T, Andrews KT. Int J Parasitol Drugs Drug Resist 2015, 5, 117-126.
Lysine acetylation in sexual stage malaria parasites is a target for antimalarial small molecules. Trenholme K, Marek L, Duffy S, Pradel G, Fisher G, Hansen FK, Skinner-Adams TS, Butterworth A, Ngwa CJ, Moecking J, Goodman CD, McFadden GI, Sumanadasa SD, Fairlie DP, Avery VM, Kurz T, Andrews KT. Antimicrob Agents Chemother 2014, 58, 3666-3678.
Towards isozyme-selective HDAC inhibitors for interrogating disease. Gupta P, Reid RC, Iyer A, Sweet MJ, Fairlie DP. Curr Top Med Chem. 2012, 12, 1479-1499.
HDAC inhibitors: modulating leukocyte differentiation, survival, proliferation and inflammation. Sweet MJ, Shakespear MR, Kamal NA, Fairlie DP. Immunol Cell Biol. 2012, 90, 14-22.
Comparative gene expression profiling of P. falciparum malaria parasites exposed to three different histone deacetylase inhibitors. Andrews KT, Gupta AP, Tran TN, Fairlie DP, Gobert GN, Bozdech Z. PLoS One 2012, 7, e31847.
Towards histone deacetylase inhibitors as new antimalarial drugs. Andrews KT, Tran TN, Fairlie DP. Curr Pharm Des. 2012, 18, 3467-3479.
Antimalarial activity of the anticancer histone deacetylase inhibitor SB939. Sumanadasa SD, Goodman CD, Lucke AJ, Skinner-Adams T, Sahama I, Haque A, Do TA, McFadden GI, Fairlie DP, Andrews KT. Antimicrob Agents Chemother. 2012, 56, 3849-3856.
HDAC inhibitors: modulating leukocyte differentiation, survival, proliferation and inflammation. Sweet MJ, Shakespear MR, Kamal NA, Fairlie DP. Immunol Cell Biol. 2012, 90, 14-22.
Histone deacetylases as regulators of inflammation and immunity. Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Trends Immunol. 2011, 32, 335-343.
Inhibitors of histone deacetylases in class I and class II suppress human osteoclasts in vitro. Cantley MD, Fairlie DP, Bartold PM, Rainsford KD, Le GT, Lucke AJ, Holding CA, Haynes DR. J Cell Physiol. 2011, 226, 3233-3241.
Ex Vivo Activity of Histone Deacetylase Inhibitors against Multidrug-Resistant Clinical Isolates of Plasmodium falciparum and P. vivax. Marfurt J, Chalfein F, Prayoga P, Wabiser F, Kenangalem E, Piera KA, Fairlie DP, Tjitra E, Anstey NM, Andrews KT, Price RN. Antimicrob Agents Chemother. 2011, 55, 961-6.
Synthesis of the Thiazole-Thiazoline Fragment of Largazole Analogues. Diness F, Nielsen DS, Fairlie DP. J Org Chem. 2011, 76, 9845-51.
Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Iyer A, Fenning A, Lim J, Le GT, Reid RC, Halili MA, Fairlie DP, Brown L. Br. J. Pharmacol. 2010, 159, 1408-1417.
Inflammatory lipid mediators in adipocyte function and obesity. Iyer, A.; Fairlie, D. P.; Prins J.; Hammock B. D.; Brown, L. Nature Reviews Endocrinology 2010, 6, 71-82.
Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. Halili MA, Andrews MR, Labzin LI, Schroder K, Matthias G, Cao C, Lovelace E, Reid RC, Le GT, Hume DA, Irvine KM, Matthias P, Fairlie DP, Sweet MJ. J Leukoc Biol. 2010, 87, 1103-1114.
Antimalarial Histone Deacetylase Inhibitors Containing Cinnamate or NSAID Components. Wheatley NC, Andrews KT, Tran TL, Lucke AJ, Reid RC, Fairlie DP. Bioorg. Med. Chem. Lett. 2010, 20, 7080-7084.
Inhibitors Selective For HDAC6 In Enzymes and Cells. Gupta PK, Reid RC, Liu L, Lucke AJ, Broomfield SA, Andrews MR, Sweet MJ, Fairlie DP. Bioorg. Med. Chem. Lett. 2010, 20, 7067-7070.
Histone deacetylase inhibitors in inflammatory disease. Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Curr. Top. Med. Chem. 2009, 9, 309-319.
Potent new antimalarial agents that cause histone hyperacetylation in Plasmodium falciparum. Andrews, K.T.; Tran, T.N.; Lucke, A.J.; Kahnberg, P.; Le, G.T.; Boyle, G.M.; Gardiner, D.L.; Skinner-Adams, T. S.; Fairlie, D.P. Antimicrobial Antiinfectives Chemother. 2008, 52, 1454-1461.
Design, Synthesis, Potency and Cytoselectivity Of Anticancer Agents Derived By Parallel Synthesis From alpha-Aminosuberic Acid. Kahnberg, P.; Lucke, A.J.; Glenn, M.P.; Boyle, G.M.; Tyndall, J.D.A.; Parsons, P.G.; Fairlie, D. P. J. Med. Chem. 2006, 49, 7611-7622.
Anti-Proliferative And Phenotype-Transforming Antitumor Agents Derived From Cysteine. Glenn, M. P.; Kahnberg, P.; Boyle, G. M.; Hansford, K. A.; Hans, D.; Martyn, A. C.; Parsons, G. P.; Fairlie, D. P. J. Med. Chem. 2004, 47, 2984-2994.
Other Enzyme Inhibitors
Quinazolinone derivatives as inhibitors of homologous recombinase RAD51.XX Ward A, Dong L, Harris JM, Khanna KK, Al-Ejeh F, Fairlie DP, Wiegmans AP, Liu L. Bioorg Med Chem Lett 2017, 27, 3096-3100.
Peptide inhibitors of the Escherichia coli DsbA oxidative machinery essential for bacterial virulence. Duprez W, Premkumar L, Halili MA, Lindahl F, Reid RC, Fairlie DP, Martin JL. J Med Chem. 2015, 58, 577-587.
Small molecule inhibitors of disulfide bond formation by the bacterial DsbA-DsbB dual enzyme system. Halili MA, Bachu P, Lindahl F, Bechara C, Mohanty B, Reid RC, Scanlon MJ, Robinson CV, Fairlie DP, Martin JL. ACS Chem Biol 2015,10, 957-64.
Virtual Screening of Peptide and Peptidomimetic Fragments Targeted to Inhibit Bacterial Dithiol Oxidase DsbA.Duprez W, Bachu P, Stoermer MJ, Tay S, McMahon RM, Fairlie DP, Martin JL. PLoS One 2015, 10(7), e0133805.
Crystal Structure of the Dithiol Oxidase DsbA Enzyme from Proteus Mirabilis Bound Non-Covalently to an Active Site Peptide Ligand.Kurth F, Duprez W, Premkumar L, Schembri M, Fairlie DP, Martin JL. J Biol Chem 2014, 289, 19810-19822.
Comparative sequence, structure and redox analyses of Klebsiella pneumoniae DsbA show that anti-virulence target DsbA enzymes fall into distinct classes. Kurth F, Rimmer K, Premkumar L, Mohanty B, Duprez W, Halili MA, Shouldice SR, Heras B, Fairlie DP, Scanlon MJ, Martin JL. PLoS One 2013, 8(11), e80210.
Rv2969c, essential for optimal growth in Mycobacterium tuberculosis, is a DsbA-like enzyme that interacts with VKOR-derived peptides and has atypical features of DsbA-like disulfide oxidases. Premkumar L, Heras B, Duprez W, Walden P, Halili M, Kurth F, Fairlie DP, Martin JL. Acta Crystallogr D Biol Crystallogr 2013, 69, 1981-1994.

